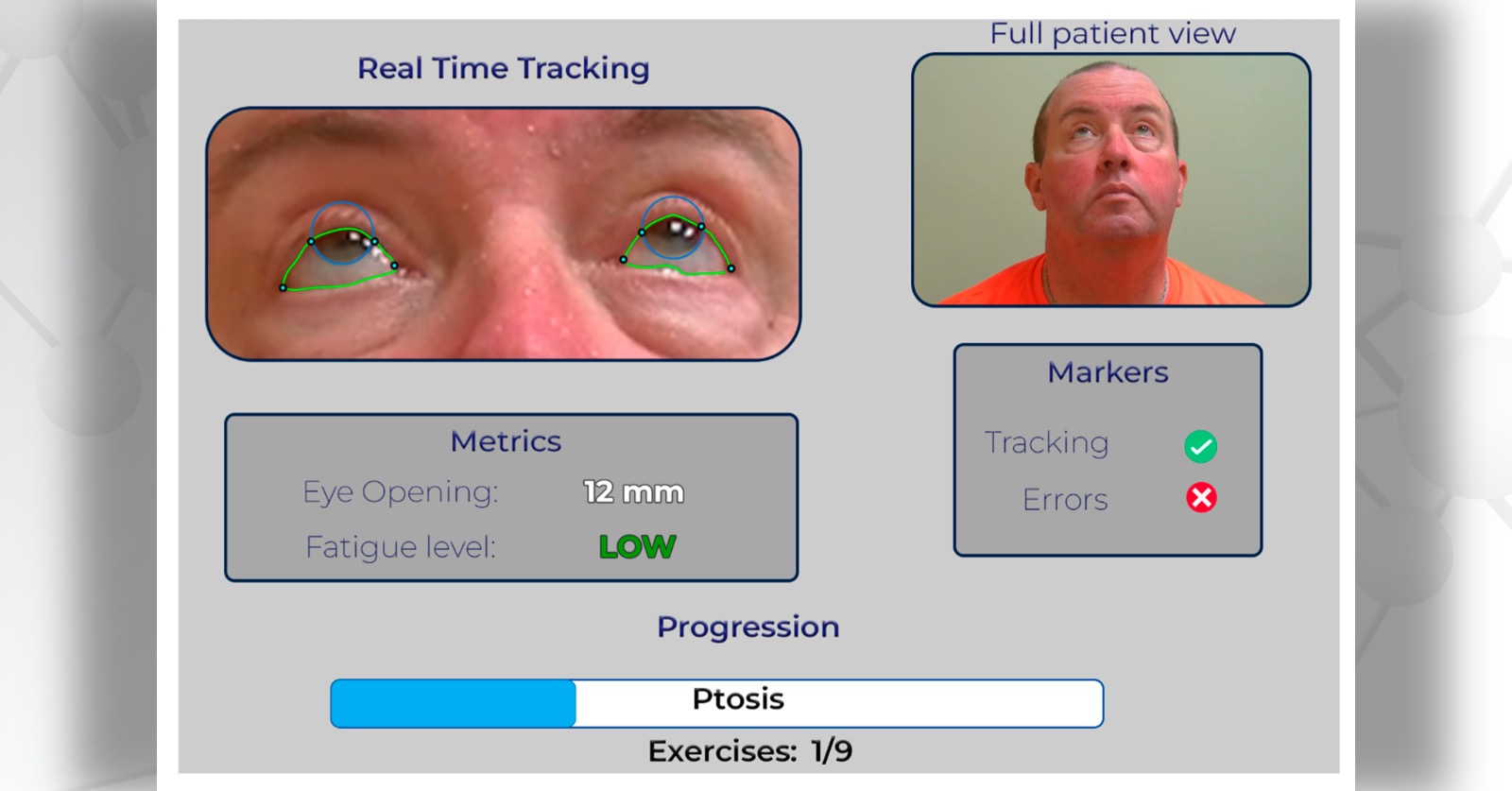
Every day, more and more patients with the neuromuscular disorder myasthenia gravis (MG) are participating in telemedicine visits. During these video appointments, neurologists can provide care that used to require an in-person visit, including evaluation of MG symptoms. But there are limitations to these remote assessments—scores vary based on human subjectivity. How can we improve the reliability of telemedicine examinations?
Researchers from the Myasthenia Gravis Rare Disease Network (MGNet) harnessed artificial intelligence to develop a new, automated tool for neuromuscular assessments in MG. Their findings, published in the journal Bioengineering on September 20, 2024, demonstrate improved scoring efficiency and accuracy to enhance patient care.
“Telemedicine is a crucial focus for MG due to its ability to overcome geographical barriers and improve accessibility to specialized care, particularly in regions where neurologists are scarce,” says lead study author Quentin Lesport, MS, of the University of La Rochelle, France. “By eliminating the need for travel and in-person appointments, telemedicine alleviates the burden on both patients and clinicians, especially for those with MG who may experience fatigue or other symptoms that make traveling difficult. However, we wanted to address the inherent subjectivity and lack of reproducibility in existing neurological assessment measures.”
The research team set to work on developing a tool that could more precisely and consistently evaluate the severity of MG. To automate the Myasthenia Gravis Core Exam (MG-CE) process, researchers used computer vision, deep learning, and natural language processing methods. A dataset of over 100 videos with 51 MG patients as well as healthy control subjects served as the foundation for training and validating the methods.
The tool works by following a three-step process, starting with extraction of individual test segments from the clinical examination videos. Next, specialized software computes the MG score for each segmented test. Finally, a graphic user interface allows clinicians to review and validate the results.
“By automating the assessment process, we can streamline the examination and allow clinicians to focus on patient care and diagnosis,” says Lesport. “These objective measures and elements enable a more efficient review of the process and results.”
One of the most promising features of the tool is its ability to effectively analyze basic telehealth videos, even when captured in uncontrolled environments. Lower-quality videos can still provide valuable insight on how a patients’ symptoms are changing over time.
“Our research aims to revolutionize the care experience for patients with MG,” says Lesport. “The ability to collect and analyze detailed patient data facilitates tailored treatment approaches. Through more frequent and consistent monitoring, early detection of exacerbations becomes feasible, enabling proactive management and reducing the burden on patients.”
Next, the team will continue to refine and finalize their methods, ensuring they are accessible and user-friendly for both patients and clinicians. The team is also exploring the tool’s applicability to other neurological diseases, including amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and post-stroke care.
“Ultimately, these advancements can improve the overall quality of life for patients by providing more accessible, efficient, and effective care,” says Lesport. “By broadening the scope of our research, we hope to further demonstrate the potential of our telemedicine-based assessment tools and contribute to the advancement of neurological care.”
Read the full study in the journal Bioengineering.
Learn more about the Myasthenia Gravis Rare Disease Network (MGNet).
The Myasthenia Gravis Rare Disease Network (MGNet) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). MGNet is funded under grant number U54NS115054 as a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS).